ISU RESEARCH ETHICS BOARD
ireb
-
ABOUT
-
SERVICES
-
FUNCTIONS
-
DOWNLOADABLES
INSTITUTIONAL RESEARCH ETHICS BOARD (IREB)
VISION
To become a globally and highly competent institutional ethics review board that will serve as a model in the ethical review of all researches to maximize safety of human subjects in the conduct of researches in the university system.
MISSION
- To promote the culture of ethics in research.
- To provide comprehensive ethical principles, rules and guidelines in the conduct of research.
- To perform a timely review of research protocols proposed by competent professionals, to ensure their ethical soundness in accordance to international statutes and guidelines of research.
OBJECTIVES
- To deliver ethically-sound review of research proposals.
- To participate in accredited seminar and workshops in research ethics locally and globally.
- To participate in the education, training and information-dissemination on the ethical conduct of scientific research.
CORE VALUES: PRAISE
- Professionalism as a prime virtue in all undertakings
- socially Responsive research outputs
- Critical and Analytical in decision making
- Moral ascendancy and Integrity as a way of life
- practice Stewardship at all cost
- Pursuit of Excellence all the time
SERVICES OF THE ISU-IREB
Institutional Research Ethics Boards (IREBs) provide a range of services to researchers, institutions, and the broader research community to ensure the ethical conduct of research involving human subjects as it highlights the diverse roles and responsibilities of a Research Ethics Board in safeguarding the rights and well-being of research participants and upholding ethical standards in research practices, namely:
1. These services emphasize the critical role of a Research Ethics Board in promoting ethical research practices, protecting research participants, and upholding the highest standards of integrity in the research enterprise.
2. These services collectively support the ethical conduct of research and help researchers navigate complex ethical issues to promote the responsible and respectful treatment of human subjects in research.
2. These services reflect the diverse roles and responsibilities of a Research Ethics Board in promoting ethical research practices, protecting the rights of research participants, and upholding the integrity of the research enterprise.
Here are some of the possible services offered by a Research Ethics Board:
1. Protocol Review: The IREB conducts thorough reviews of research protocols submitted by researchers to ensure compliance with ethical guidelines and standards.
2. Consultation Services: The IREB may offer consultation services to researchers, guiding on ethical issues, protocol development, informed consent procedures, and other aspects of research ethics.
3. Educational Workshops and Training: The REB may organize workshops, seminars, and training sessions for researchers, staff, and students on research ethics, regulatory requirements, and best practices in human subjects research.
4. Pre-Submission Reviews: The IREB may offer pre-submission reviews to researchers, providing feedback on draft protocols to help ensure they meet ethical standards before formal submission.
5. Informed Consent Templates and Guidance: The REB may provide researchers with standardized informed consent templates and guidance on obtaining valid and ethically sound informed consent from research participants.
6. Regulatory Compliance Assistance: The REB helps researchers navigate regulatory requirements related to research ethics, assisting with compliance with local, national, and international regulations.
7. Conflict of Interest Management: The REB may guide managing and disclosing conflicts of interest that may arise in research activities.
8. Monitoring and Auditing: The REB may conduct monitoring visits or audits of research projects to ensure ongoing compliance with ethical standards and regulatory requirements.
9. Policy Development and Review: The REB may develop, review, and update institutional policies and procedures related to research ethics, ensuring alignment with current regulations and best practices.
10. Community Engagement: The REB may facilitate community engagement activities to involve stakeholders in the research process and ensure that research activities align with community values and interests.
11. Research Participant Advocacy: The REB may serve as an advocate for research participants, ensuring that their rights, safety, and well-being are protected throughout the research process.
12. Response to Ethical Concerns and Allegations: The REB investigates and responds to ethical concerns raised by participants, researchers, or others, addressing any alleged violations of ethical standards promptly and appropriately.
13. Review of Amendments: The REB reviews and approves any proposed amendments or modifications to approved research protocols to ensure that changes maintain ethical standards.
14. Post-Approval Monitoring: The REB may conduct post-approval monitoring of research projects to ensure that researchers are adhering to approved protocols and ethical guidelines throughout the study.
15. Adverse Event Reporting: The REB establishes procedures for researchers to report adverse events or unanticipated problems that occur during the course of the research, ensuring appropriate responses to protect participants.
16. Data and Safety Monitoring: The REB may oversee data and safety monitoring plans for research studies involving significant risks to participants, ensuring that data integrity and participant safety are maintained.
17. Collaboration with External Agencies: The REB may collaborate with external agencies, such as regulatory bodies, funding agencies, or ethics committees from other institutions, to facilitate multi-site research projects and ensure consistent ethical oversight.
18. International Research Support: For studies involving international collaboration or conducted in multiple countries, the REB may guide navigating cultural differences, regulatory requirements, and ethical considerations across different jurisdictions.
19. Policy Interpretation and Guidance: The IREB interprets institutional policies, regulatory guidelines, and ethical standards for researchers, guiding how to apply these principles in specific research contexts.
20. Research Ethics Training for Committee Members: The REB offers training and professional development opportunities for its members to ensure they are equipped with the knowledge and skills necessary to fulfill their roles effectively.
21. Quality Improvement Initiatives: The IREB may initiate quality improvement projects to enhance the efficiency and effectiveness of the ethics review process, streamline procedures, and promote continuous improvement in ethical oversight.
22. Research Integrity Support: The IREB may provide support and guidance to researchers on issues related to research integrity, including data management, publication ethics, and responsible conduct of research.
23. Research Ethics Policy Development: The IREB may be involved in developing and revising institutional research ethics policies to ensure alignment with current ethical standards, regulatory requirements, and best practices in research ethics. By staying up-to-date with evolving ethical considerations, the REB can contribute to the development of robust policies that guide ethical conduct in research.
24. Public Engagement and Outreach: The IREB may engage with the public through outreach activities, such as informational sessions, public forums, or community events, to raise awareness about research ethics and the importance of protecting human subjects.
25. Collaboration with other Research Compliance Offices of the university as well as other institutions outside the university: The IREB may collaborate with research compliance offices within the institution to ensure coordinated oversight of research activities, particularly concerning ethical, regulatory, and compliance issues. By working closely with research compliance offices, the REB can help ensure that research activities adhere to institutional policies and external regulations, promoting a culture of research integrity and ethical conduct.
FUNCTIONS OF THE ISU-RESEARCH ETHICS BOARD
A Research Ethics Board (REB), plays a crucial role in overseeing and ensuring the ethical conduct of research involving human subjects. These functions collectively serve to uphold the ethical principles of respect for persons, beneficence, and justice in research involving human subjects.
Here are some of the key functions of a Research Ethics Board:
1. Reviewing Research Protocols: The primary function of an REB is to review research protocols submitted by researchers to ensure that the proposed research meets ethical standards and guidelines.
2. Protecting Human Subjects: The REB ensures that the rights, safety, and well-being of human subjects participating in research are protected. This includes assessing the risks and benefits of the research to participants.
3. Informed Consent: The REB ensures that informed consent is obtained from research participants. This involves ensuring that participants are fully informed about the research, including its purpose, procedures, risks, and benefits, and that they voluntarily agree to participate.
4. Continuing Review: The REB conducts periodic reviews of ongoing research to ensure that ethical standards are maintained throughout the study.
5. Conflict of Interest Management: The REB helps manage and mitigate conflicts of interest that may arise in the conduct of research, particularly concerning the researchers and the institution.
6. Education and Training: The REB may provide education and training to researchers, staff, and students on ethical principles and guidelines for conducting research involving human subjects.
7. Policy Development: The REB may develop and update policies and procedures related to research ethics, ensuring that they align with current regulations and best practices.
8. Monitoring and Compliance: The REB monitors research activities to ensure compliance with ethical standards, regulations, and institutional policies.
9. Responding to Concerns and Violations: The REB addresses concerns raised by participants, researchers, or others regarding the ethical conduct of research and investigates any alleged violations of ethical standards.
10. Reporting and Documentation: The REB maintains records of its reviews, decisions, and communications related to research protocols, ensuring transparency and accountability in the review process.
11. Risk Assessment: The REB assesses the potential risks associated with the research to ensure that adequate measures are in place to protect the participants from harm.
12. Confidentiality and Data Protection: The REB ensures that researchers have appropriate plans in place to protect the confidentiality of participants' data and to secure any personal information collected during the research.
13. Review of Recruitment and Consent Procedures: The REB evaluates the methods used by researchers to recruit participants and obtain their consent to participate in the research to ensure they are appropriate and ethical.
14. Review of Research Instruments and Materials: The REB may review research instruments, surveys, questionnaires, and other materials used in the study to ensure they are appropriate and do not pose undue risks to participants.
15. Review of Conflict Resolution Procedures: The REB may review and approve procedures for resolving conflicts that may arise during the research process, particularly regarding participant welfare and rights.
16. International Research Oversight: In cases where research involves international collaboration or is conducted in multiple countries, the REB may coordinate with other ethics boards to ensure that ethical standards are met across different jurisdictions.
17. Community Engagement: The REB may promote community engagement in research by ensuring that community interests are considered and respected in the research process, particularly in studies involving vulnerable populations or sensitive topics.
18. Evaluation of Research Impact: The REB may assess the potential impact of the research on participants, communities, and society to ensure that the benefits of the research outweigh any potential risks.
19. Communication and Feedback: The REB communicates its decisions and recommendations to researchers on time, providing feedback and guidance for addressing any ethical issues identified during the review process.
20. Quality Assurance: The REB plays a role in ensuring the overall quality and integrity of research conducted within the institution by upholding ethical standards and promoting responsible conduct in research.
SCOPE OF POLICIES
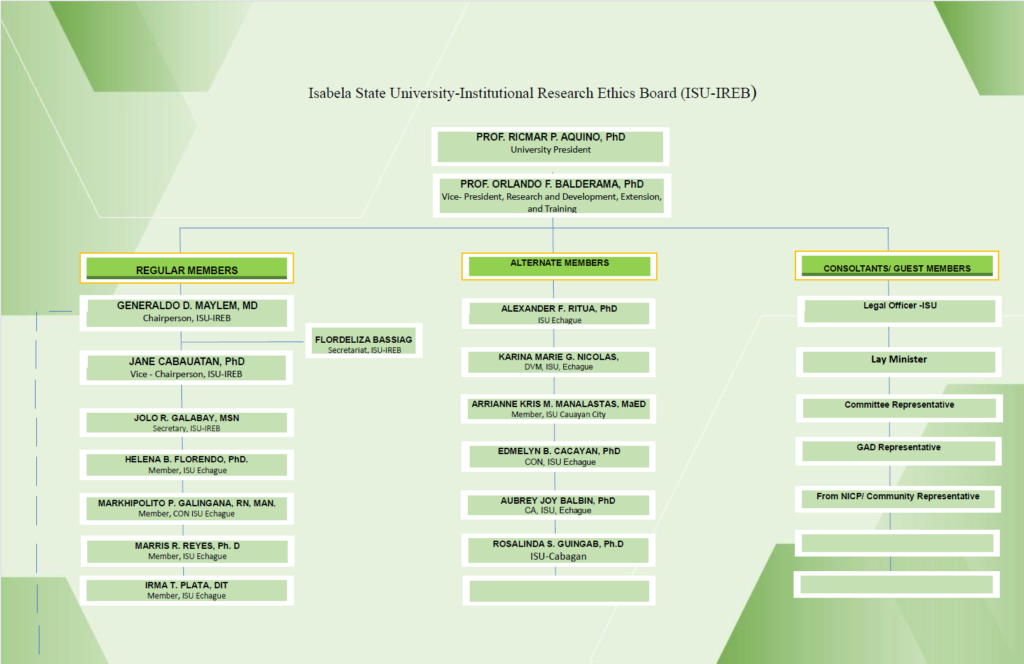
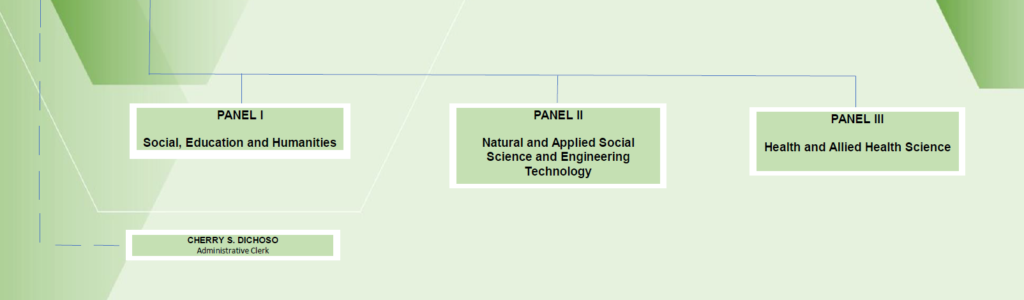
ORGANIZATIONAL CHART